Evidence
Transparency
Technology
CLINICAL TRIALS
RESEARCH CENTERS
PATIENTS IN RANDOMIZED CONTROLLED TRIALS (RCT)
Published
TWO SYSTEMATIC REVIEWS
WITH META-ANALYSES
RCT ARTICLES
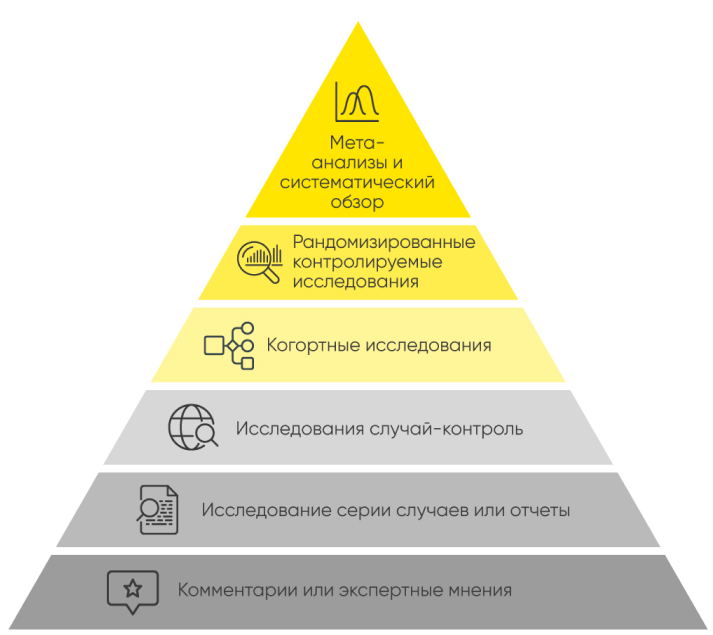
Efficacy and safety of our medications are studied in randomized placebo-controlled trials, and in systematic reviews with meta-analyses.
A randomized clinical trial is a way to study therapeutic effects (often, of medications), in which participants are randomly allocated among the treatment and control groups. Randomization eliminates bias in treatment assignment. The control group receives standard therapy or a placebo (a sham substance or treatment which is designed to have no known therapeutic value). Additionally, participants can be blinded to the treatment allocations, i.e., information about the prescription of a particular therapy can be withheld from the patients and physicians involved, which reduces bias in the evaluation of the results in the experimental and control groups.
Double-blind randomized placebo-controlled clinical trials are the “gold standard” for studying the efficacy and safety of medications. And cumulative evaluation of the findings of multiple RCTs in the form of a systematic review with meta-analysis is the top level of evidence-based medicine.
The designs of and reports on ongoing and completed clinical trials are available at ClinicalTrials.gov of the U.S. National Institutes of Health. The results of clinical trials, after undergoing an independent peer review procedure, are published in Russian and foreign scientific journals.
Read more
The Interactive Voice System: Our Company uses an electronic randomization system to randomize patients and allocate packages of the studied medication. The system makes it possible to ensure the blinding of the information about the allocation of the patients among treatment and control groups throughout the trial, as well as to avoid the influence of human factor when distributing packages to patients.
SKIFIS: To collect data in a clinical trial, we use an electronic system of remote data collection to control the timeliness of data acquisition, as well as completeness and quality of data.

Patient’s E-Diary: A patient’s e-diary is used in most of our clinical trials. That way, we receive data directly from the patient in real time. Our e-diary also allows the patient to communicate directly with the investigator—e.g., in the event the patient’s condition worsens or if they need to ask questions about the clinical trial.

Registry of issued approvals for clinical trials of medicines: trials underway
Ministry of Health of the Russian Federation
Registry of issued approvals for clinical trials of medicines: trials completed
Ministry of Health of the Russian Federation
ClinicalTrials.gov [ENG]
U.S. National Institutes of Health